Check out our paper on processing of FUBI-eS30 in eLife!
The ubiquitin-like protein FUBI and the ribosomal protein eS30 are synthesized as one polypeptide chain. In our recent eLife publication we discovered that USP36 is involved in endoproteolytic cleavage of the fusion protein; a reaction licensing efficient final maturation of 40S subunits. Congratulations to Jasmin, Caroline, Kerstin, Emanuel, Ivo and Ulli!
It is vital for every living cell to translate messenger RNA into proteins. This process is catalyzed by nanomolecular machines called ribosomes. In humans, a 40S and 60S subunit form a mature ribosome consisting of 84 different components, which have to be placed correctly within the final ribonucleoprotein particle to ensure high fidelity of protein synthesis.
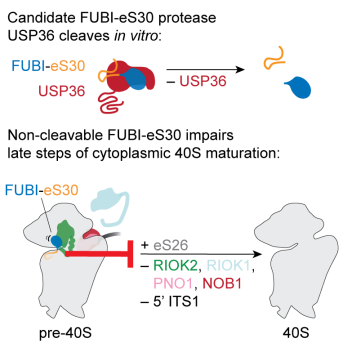
The ribosomal protein eS30 is encoded in the same gene as the ubiquitin-like protein FUBI in humans, generating a linker-free fusion protein. While eS30 is part of mature 40S subunits, FUBI is cleaved off by a yet unknown enzyme. Using a combination of biochemical, cell biological and proteomic approaches, researchers of the Kutay lab at IBC gained mechanistic insight into the importance of endoproteolytic FUBI-eS30 processing for ribosome biogenesis.
Expression of non-cleavable FUBI-eS30 constructs in human cells affected late steps of cytoplasmic 40S subunit maturation, manifesting in impaired 18S pre-rRNA processing and recycling of certain ribosome biogenesis factors. Comparison of proteins co-purified with wild-type or non-cleavable FUBI-eS30 identified the nucleolar deubiquitinase USP36 as a candidate FUBI-eS30 protease. RNAi- or CRISPRi-mediated USP36 depletion as well as in vitro processing assays confirmed its active role in FUBI-eS30 cleavage. Together, these results highlight the functional importance of FUBI-eS30 processing and with USP36, van den Heuvel et al. identified a new player in this pathway.