Structure and regulation of the microtubule plus-end tracking protein Kar9
In Structure, the Barral (IBC) and Steinmetz (PSI) labs provide structural insights in the microtubule-tracking protein Kar9, revealing how regulation of its dimerization orients the mitotic spindle in dividing cells. This study uncovers Kar9’s relationships to metazoan MACF/ACF7/Shot and PRC1.
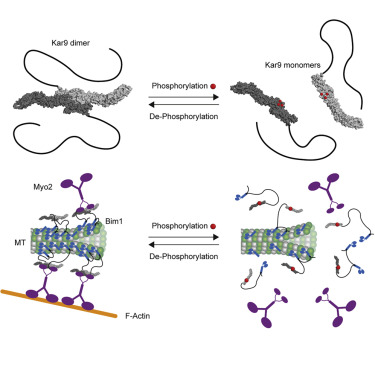
In eukaryotes, the spatial coordination of chromosome segregation with cell cleavage relies on the interaction of selected microtubules with the actin cytoskeleton through dedicated microtubule plus-end tracking proteins (+TIPs), such as the yeast protein Kar9. Relaxing the selectivity of these +TIPs localization randomizes spindle positioning and may cause massive chromosome loss. Indeed, the restricted localization of Kar9 to the microtubules of only one spindle aster drives alignment of the mitotic spindle with the division axis of the budding yeast cell.
The crystal structure of Kar9’s folded domain reveals that it is formed of three spectrin repeats reminiscent of the +TIPs MACF/ACF7/Shot and PRC1/Ase1. Point mutations abrogating spectrin-mediated dimerization of Kar9 in vitro, reduce and randomize Kar9 distribution to microtubule tips in vivo, impairing spindle positioning. Strikingly, the structure reveals that six phosphorylation sites targeted by the Cdk1 kinase precisely surround the Kar9 dimerization interface. Phosphomimetic substitution of these sites inhibit Kar9 dimerization, displac Kar9 from microtubules and prevent its proper interaction with the actin cytoskeleton. Our results provide molecular-level understanding on how diverse cell types may regulate and precisely pattern in space the interaction of their microtubules with the actin cytoskeleton such as to properly orient and orchestrate processes like cell division, cell growth and cell motility.
external page Full paper in Structure