Research
Cell size and DNA content
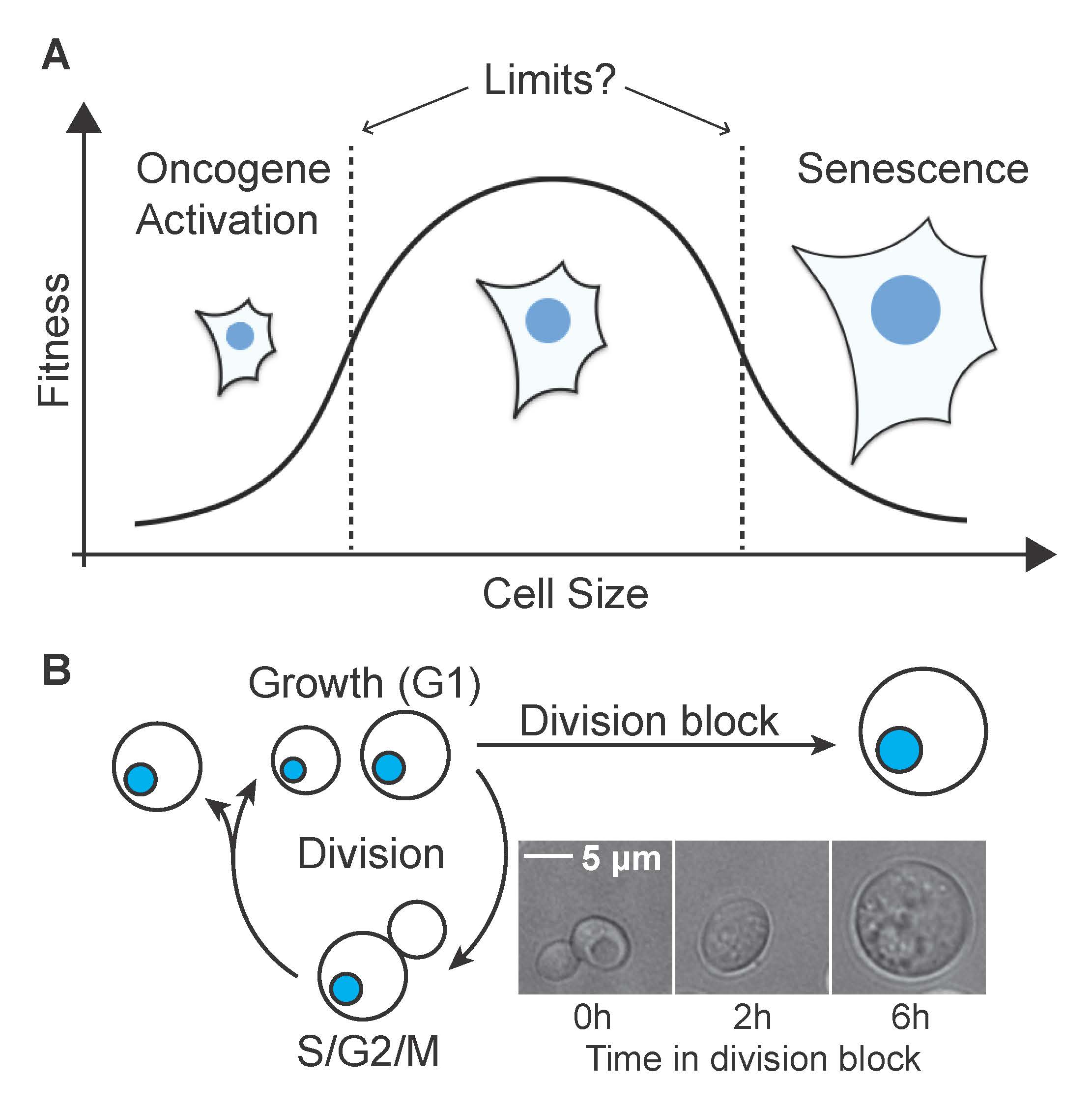
All cells of a given cell type have the same size and loss of cell size homeostasis correlates with altered cell function: During cell senescence cells grow very large and activation of certain oncogenes results in premature cell cycle entry at an unnaturally small size. This suggests that having a specific cell size is critical for cell function. But what defines the upper and lower size limits within which cells can function normally (Figure 1A)?
Figure 1 A) The fact that all cells regulate their size suggests that cell size is under evolutionary constraint. The fitness costs associated with deviation from normal cell size are not known. B) A system to experimentally manipulate cell size: Cell growth (biomass accumulation) and cell division are only loosely coupled. Blocking cell cycle progression does not stop biomass accumulation and arrested cells continue to increase in size.
DNA content limits maximal cell size:
To determine what limits maximal cell size, we have previously developed a system that allows generation of yeast and human cells that are up to 10-fold bigger than cycling cells (Figure 1B). This excessive increase in cell size leads to a breakdown of multiple cell functions: Oversized cells proliferate slowly and senesce prematurely, harbor high levels of DNA damage and fail to respond to external cues. Increasing DNA content in large cells suppresses size associated defects, demonstrating that DNA content becomes limiting when cells grow too large. In oversized cells, RNA and protein synthesis are attenuated, but cell volume continues to increase, resulting in a diluted cytoplasm (Figure 3, Neurohr et al. 2019). This surprising finding demonstrates that protein synthesis and cell volume increase are regulated to some degree independent from each other. We are currently investigating how large size accelerates cell senescence and how cytoplasm density affects cell function.
Why do cells need a minimal size?
While cells can grow very large during cell senescence, cell size control mechanisms normally ensure that cells do not enter cell division prematurely at an unnaturally small size. Loss of cell size homeostasis and premature cell cycle entry correlate with DNA replication stress and occur for example as a result of oncogene activation. We use genetic manipulations and quantitative live microscopy to systematically determine how critically small size affects cell fitness in budding yeast and tissue culture cells.
Role of cell size in Senescence
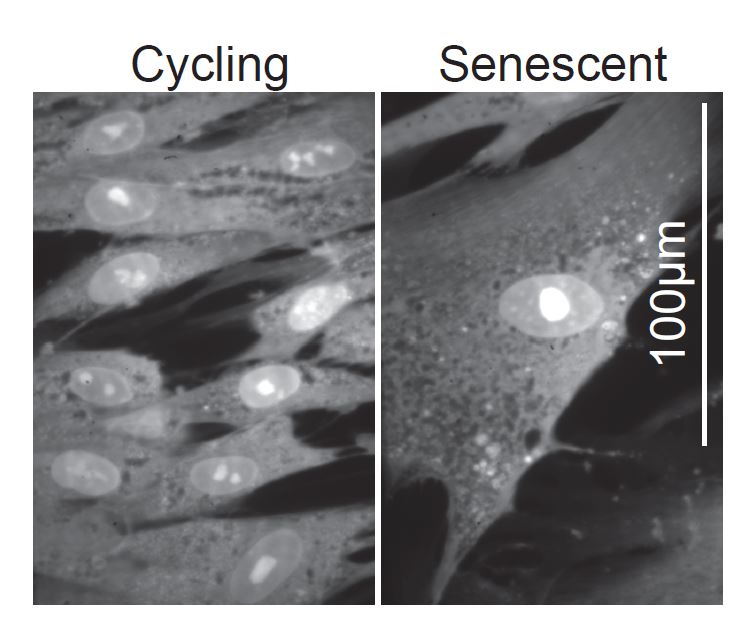
Senescent human fibroblast cells grow several times larger than cycling cells.
Aging affects most organisms and is characterized by a progressive loss of function over time. Cell senescence plays a key role in aging of both, unicellular and multicellular organisms. A conserved characteristic of senescent cells is that they become very large and aged yeast cells display a very similar set of defects as oversized cells (Neurohr et al. 2018, Neurohr et al. 2019), indicating that the increased cell size that accompanies cell senescence actively contributes to loss of cell function. Importantly, increasing cell size in both budding yeast and human cell lines accelerates replicative aging and senescence (Neurohr et al. 2019). We are investigating how increased cell size affects the molecular networks that regulate replicative aging in budding yeast and cell senescence in human cells.
Cell density
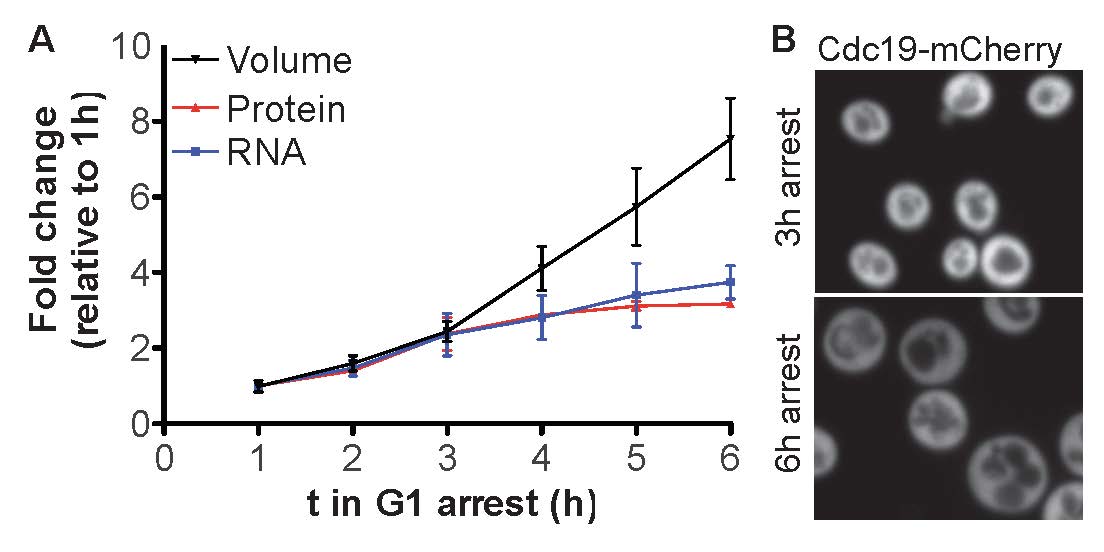
The cytoplasm of large cells is less dense. A) Quantification of cell volume and total cellular RNA and protein content as cells grow large during a prolonged cell cycle arrest. B) Confocal microscopy images of small and large cells expressing an abundant cytoplasmic protein: the cytoplasm of large cells is diluted.
The tight packing of macromolecules in the cytoplasm generates unique biophysical properties inside the cell. Changes in cell density are therefore expected to have far reaching functional consequences for cell physiology. Not surprisingly, cell density is very narrowly distributed among a given population of cells. Cell density however fluctuates during cell division and can change during differentiation, starvation, apoptosis and cell senescence (Neurohr et al. 2020). How density is regulated and how cytoplasm density affects cellular functions is largely unknown. We have previously discovered that cytoplasm density decreases when cells grow too large (Neurohr et al. 2019), because protein synthesis is attenuated while cell volume continues to increase (Figure 3A). Cell volume increase is therefore not simply a passive consequence of protein synthesis, but cell volume increase and macromolecule synthesis are to some degree regulated independent from each other. We want to understand how macromolecule biosynthesis and cell volume are coordinated in order to maintain intracellular protein concentration constant. For this purpose, we are using budding yeast to investigate A) How cell size affects macromolecule biosynthesis, B) How the regulatory networks that govern cell volume increase and protein synthesis are coordinated and C) How cytoplasm density affects basic cellular processes such as transcription initiation and signaling.
Relevant Publications
Neurohr GE, Terry RL, Lengefeld J, Bonney M, Brittingham GP, Moretto F, Miettinen TP, Pontano Vaites L, Soares LM, Paulo J, Harper W, Buratowski S, Manalis S, van Werven FJ, Holt LJ, Amon A. Excessive cell growth causes cytoplasm dilution and contributes to senescence. Cell. 2019; 176, 1083-1115.
Neurohr GE, Terry RL, Sandikci A, Zou K, Li H, Amon A. Deregulation of the G1/S-phase transition is the proximal cause of mortality in old yeast mother cells. Genes & Development. 2018; 32, 1075-1084.
Neurohr GE and Amon A, Relevance and Regulation of Cell Density (Review). Trends in Cell Biol, 2020; 30(3):213-225.