Nuclear protein homeostasis
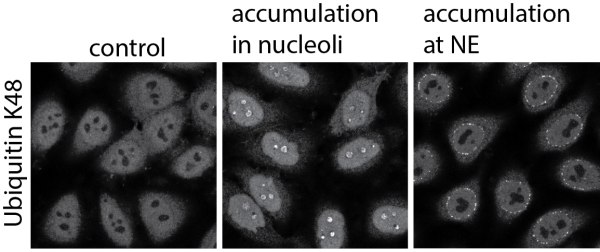
Over many years, the biogenesis of nuclear components has been intensively studied. In comparison, we still know little about how protein homeostasis (proteostasis) is controlled in the nuclear compartment. Although the cell nucleus is not a protein folding compartment, the nucleus contains a versatile suite of proteostatic factors including many molecular chaperones and a nuclear branch of the ubiquitin-proteosome system (UPS). Challenges to nuclear proteostasis arise from conventional stresses such as as heat or oxidative stress, error-prone nuclear biosynthetic processes (e.g. ribosome synthesis), the age-associated decline of factors belonging to the proteostatic network or mutations that give rise to ill-behaved proteins that form nuclear protein aggregates. Long-term perturbation of nuclear proteostasis can be associated with neurodegenerative diseases.
We are interested in different aspects of nuclear proteostasis, namely
• changes to the nuclear proteome upon entry into senescence,
• proteostasis at the inner nuclear membrane,
• proteostatic mechanisms governing ribosome synthesis,
• impact and fate of intranuclear protein aggregates,
and are looking for talented PhD students and post-docs as well as bioinformaticians to work on these problems.