Topic 1: Microtubule patterning in spindle positioning
The microtubule cytoskeleton plays a multitude of roles in transporting cargos (organelles, chromosomes, etc…) and organizing cellular architecture throughout the cell cycle. This requires that the function and dynamic behaviour of each individual microtubule becomes properly specified, depending on where they are and what they are supposed to do. The mechanisms of this specification are still largely mysterious.
Our Lab studies the molecular mechanisms of microtubule specification in budding yeast. We focus on two mechanisms of specification: (i) The restricted recruitment of kinesins to only specific microtubules and (ii) the liquid-like phase separation of +TIP proteins (proteins that bind and track the +end of microtubules) into bodies at the end of only few, specific microtubules.
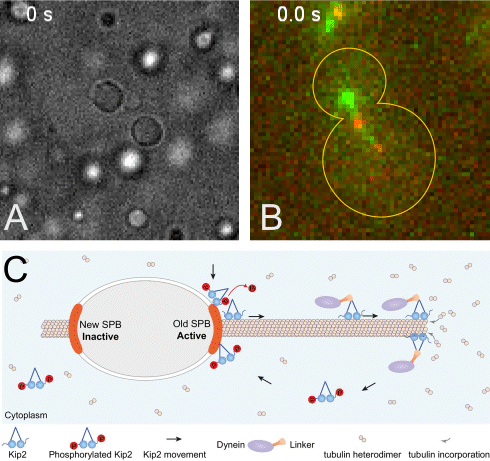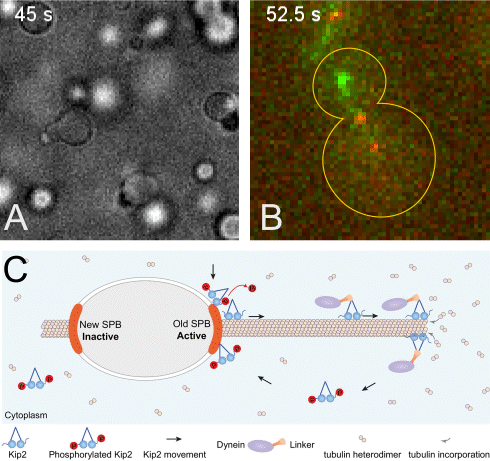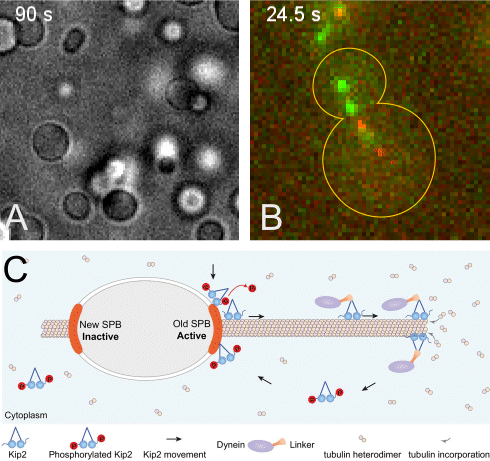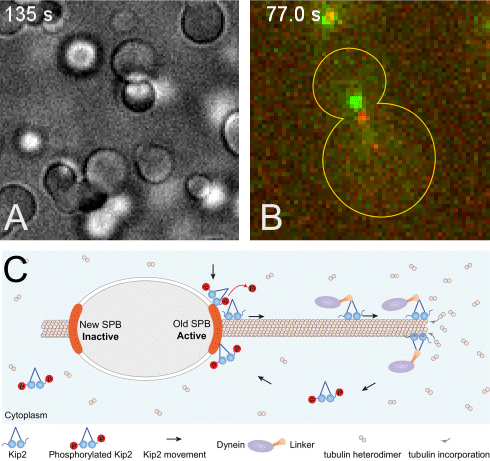
(A) The +TIP proteins (microtubule plus-end tracking proteins) Kar9, Bim1 and Bik1 form a protein network that phase separates when assembled in vitro.
(B) Kar9 localization (in green) at the plus-end of a dynamic unlabeled astral microtubule emanating from the old spindle pole body (old SPB, visualized as the more intense red focus; SPBs are the yeast equivalent of the centrosomes of animal cells). The microtubule undergoes successive phases of growth and shrinkage, while the Kar9 +TIP network persistently tracks its dynamic plus-end. Remarkably, Kar9 decorates the tip of this microtubule only, and not that of the microtubules emanating from the other SPB.
(C) Model for remote control of dynamic microtubules from their SPB-embedded minus-end. The kinesin Kip2 is recruited to the old SPB in a phosphorylation dependent manner, it translocates to the plus-end of the microtubule, where it acts as a polymerase and promotes microtubule plus-end extension and cargo/dynein delivery to the bud. Inhibition of its recruitment at the young SPB causes microtubules coming from that SPB to remain short.
Group members currently working on this topic:
Interested in a Master Project on this topic?
Highlight publications:
Contact
Institut für Biochemie
Otto-Stern-Weg 3
8093
Zürich
Switzerland
